Description
Ethylene glycol is an organic compound with the formula (CH2OH)2. This material is mainly used for two purposes as a raw material in the manufacture of polyester fibers and antifreeze formulation. It is an odorless and colorless liquid with a sweet taste. In terms of appearance, it is a viscous liquid.

The common name ethylene glycol literally means "glycol derived from ethylene".
This substance is among the most toxic compounds and eating and drinking it is very dangerous and may lead to severe illness or death.
History
Ethylene glycol was first prepared in 1856 by a French chemist Charles Adolphe Wutz. Prior to the First World War, commercial use of this compound was unheard of. It was only in 1917 that the United States of America produced ethylene glycol through ethylene chlorohydrin. In 1925 Union Carbide Corp established the first large-scale glycol producing plant in South Charleston, West Virginia. 1929 onwards this compound was used in explosives industry. Vapour-phase oxidation of ethylene to ethylene oxide was a popular process. This compound has also been found in outer space
Applications of MEG

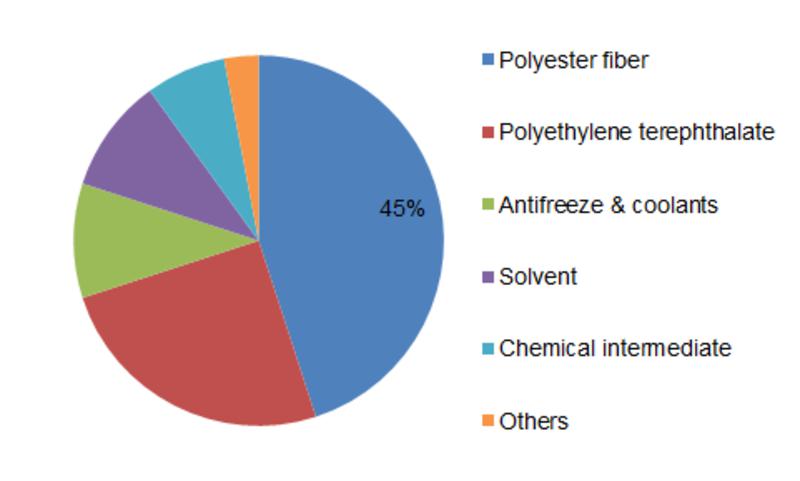
It is used as one of the raw materials for the production of antifreeze for use in car radiators.
It is also used as an ingredient in hydraulic fluids, printing inks and paint solvents.
It is also used as a reagent in the manufacture of polyesters, explosives, alkyd resins and synthetic waxes.
Ethylene glycol is used in the natural gas industry to remove water vapor from natural gas before further processing, in exactly the same way as triethylene glycol (TEG).
Monoethylene glycol (MEG) can be used for applications that require chemical intermediates.
Monoethylene glycol is also used in the production of resins, solvent binders, freezing point depressants, solvents, wetting agents and chemical intermediates.
These applications are critical to the production of a wide range of products, including resins.
In the production of water-based adhesives, various latex paints are used.
It is also used for asphalt emulsion.
It is also used to make electrolytic capacitors.
It is used in textile fibers and in the paper industry.
It is used to produce all kinds of polyester resins (fibers, dishes and films).
It is also used as a softener (glue, varnish and enamel) in the production of resin esters.
It is used in alkyd type resins (synthetic rubbers, adhesives, surface coatings).
It is used as a stabilizer against gel formation.
Used in reducing fluids (airplanes, runways) as well as heat transfer fluids (gas compressors, heating, ventilation, air conditioning, process chillers).
Medium is used for conductive salt suspension in electrolytic capacitors.
Safety aspects of Monoethylene glycol
Since the ignition point of monoethylene glycol is high (115 ᵒC), this product does not ignite normally, but it will start to burn if exposed to high heat or flames.
In case of accidental leakage of monoethylene glycol, the following safety measures should be taken:
Remove any possible sources of ignition from the area. (any smoking, oil lighting, spark or flame)
Avoid any body contact with monoethylene glycol.
Use appropriate protective clothing and equipment. To protect the eyes, use special glasses resistant to chemicals. To protect the body skin, use special protective clothing resistant to chemicals.
Monoethylene glycol market
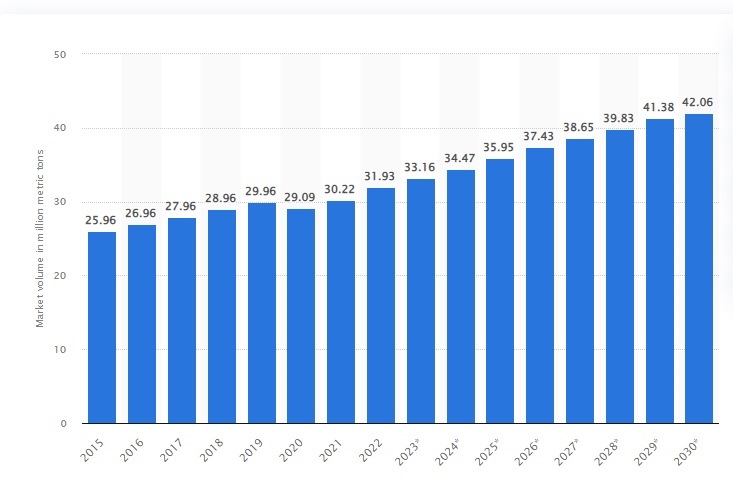
Increasing demand for antifreeze, PET, resin, and polyester fibers is the main factor driving the global monoethylene glycol market. Monoethylene glycol (MEG), a downstream petrochemical product, is mostly produced in the Middle East and Africa, with Saudi Arabia leading the list in terms of annual net exports. From a regional point of view, East Asia is known to be the largest market for monoethylene glycol (MEG) due to having a large share of the global monoethylene glycol (MEG) market. While China dominates the regional monoethylene glycol (MEG) market, South Asia, with its expanding economy, is one of the most lucrative markets for monoethylene glycol (MEG) manufacturers worldwide, with forecasts suggesting that the use of MEG, PET production will have the highest growth rate in 2023 due to the increase in the consumption of packaging materials in the food and beverage industries.
Global monoethylene glycol market volume 2015-2030
It should be noted that Iran has also succeeded in producing ethylene glycol and currently has12% of the production capacity of ethylene glycol in the Middle East. which is equivalent to 4% of the world's production capacity. China and India had the highest demand for ethylene glycol in 2014. After these two countries, the countries of Turkey, Pakistan and UAE constitute 6% of Iran's export value. Now according to published reports, Iran exports most of its exports to India and Pakistan. Iran has become the largest exporter of MEG by sending 158 shipments of this substance.
Click here to see the last 100 shipments from Iran to various destinations.
This material is available with different brands for export. It is exported to countries such as: Russia, Kazakhstan, Armenia, Turkey, etc. by Mahour Polymer Tiran Trading. Contact our business experts for more information. We will offer you the best price and as soon as possible
Packing

MEG can be sold in 220-liter polyethylene barrels, IBC tanks, and in bulk.